

Valbiopure
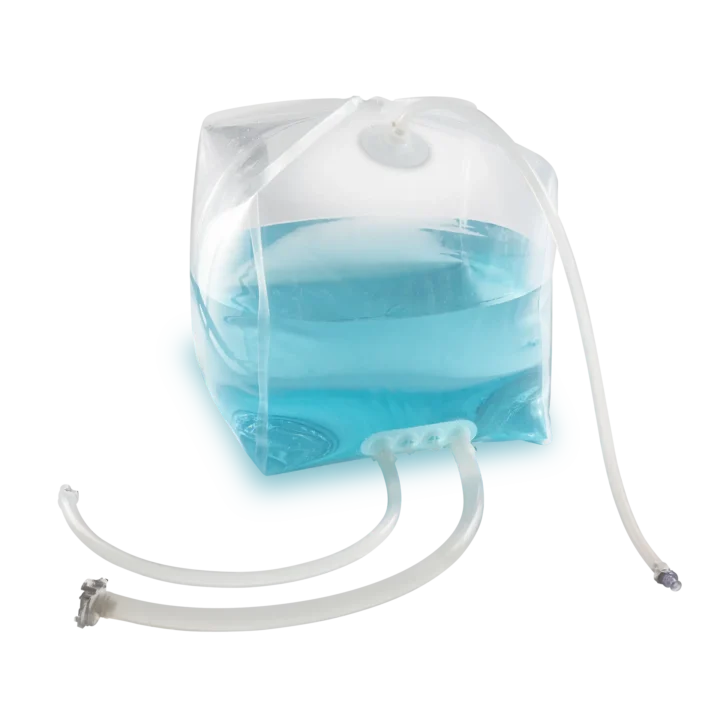
ValBiopure Single-Use Systems Bag.
Flexible, Reliable, Customizable
2D – 3D bag up to 3000 L
Manufactured in ISO 7 Cleanroom using high‑quality Renolit Infuflex® 9101 film (PE, EVOH) and Granuflex® 4301 ports (PE). Designed for safe storage and transport of fluids, fitting market‑leading totes, with strong customization options and 100% leak‑tested.
Flexible customization of sizes, tubings, joints, and filters for a variety of biopharmaceutical processes
Why choose Valbiopure?
2D Bag

100% leak test
Boat port with high configuration flexibility (HB diameter and quantity)
Sealed batch number to ensure traceability
Double wound film to minimize particulates presence
Capacity: from 5ml to 20L
Assembly available with tubes and connection based on customer needs
3D Bag
Patented K-welding system for continuous, leak-free welds
Automated system: Reliable and replicable process; Under laminar flow to reduce particulates
Laser-marked data matrix for excellent traceability
Automated dimensional control in-line
Ports can be placed in any position (main and rear side). High flexibility in diameter selection
100% leak test
Flexible dimensioning to perfectly fit your existing hardware
Capacity: from 50L to 3000L
Assembly with tubes and connection based on customer needs
Validation and Quality Assurance
- Manufactured in ISO 7 cleanroom
- Quality system in according to ISO 13485
- Sterilization by Gamma irradiation method
- 100% leak test
- The materials which compose the Valbiopure bags comply with:
- Endotoxin USP 85 / Ph. Eur. 2.6.14
- Biological reactivity test USP <87> / <88> (USP Class VI)
- Plastics USP 661.1
- Biocompatibility Tests ISO 10993-4/5/6/10/11
- Quality system in according to ISO 13485
- Gamma irradiated
- 100% 2D bags leak test. Coming soon: 3D bags leak test
- The materials which compose the Biopure bags comply with:
- Endotoxin USP 85 / Ph. Eur. 2.6.14
- Biological reactivity test USP <87> / <88> (USP Class VI)
- Plastics USP 661.1
- Biocompatibility Tests ISO 10993-4/5/6/10/11
- Qualification studies ongoing:
- Extractable Study (USP 1665, USP 665 / BPOG Guidance)
- Physico-chemical material properties and Chemical compatibility chart
- Validation tests including:
- Mechanical Tests
- Connection Integrity Test
- Gamma Sterilization, Filling and Transport Resistance
- Resistance to Freezing Test up to -80°C
- Physicochemical Testing (USP <661.2>, Ph. Eur. 2.6.14, USP <788> and USP <790>)
- Shelf-Life Evaluation (Ongoing – accelerated testing – 40°C, 75% RH and real-time testing – 25°C, 60% RH)
Bag Models 2D
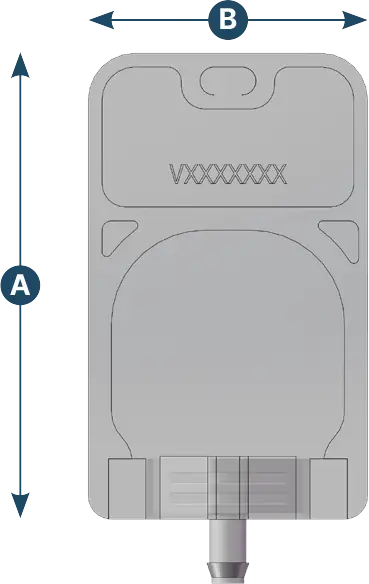
2D Baby
| Capacity (ml) | A (mm) | B (mm) | Max ports q.ty | Ports configuration |
|---|---|---|---|---|
| 5 | 100 | 60 | 1 | 1/8" - 1/4" |
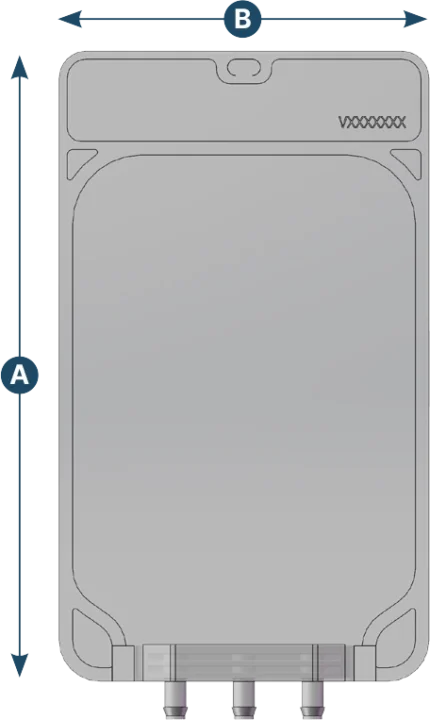
2D Medium
| Capacity (ml) | A (mm) | B (mm) | Max ports q.ty | Ports configuration |
|---|---|---|---|---|
| 50 | 150 | 100 | 3 | 1/8" - 1/4" - Closed |
| 100 | 180 | 100 | 3 | 1/8" - 1/4" - Closed |
| 250 | 230 | 135 | 3 | 1/8" - 1/4" - Closed |
| 500 | 270 | 150 | 3 | 1/8" - 1/4" - Closed |
| 1000 | 330 | 180 | 3 | 1/8" - 1/4" - Closed |
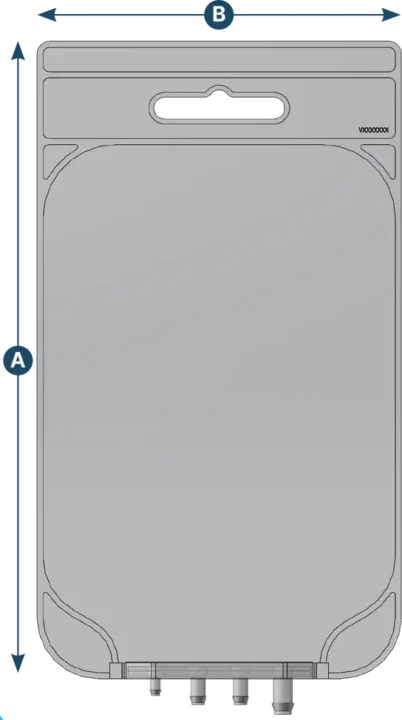
2D Big
| Capacity (ml) | A (mm) | B (mm) | Max ports q.ty | Ports configuration |
|---|---|---|---|---|
| 2 | 380 | 240 | 4 | 1/8" - 1/4" - 3/8" - 1/2" - Closed |
| 3 | 455 | 280 | 4 | 1/8" - 1/4" - 3/8" - 1/2" - Closed |
| 5 | 525 | 300 | 4 | 1/8" - 1/4" - 3/8" - 1/2" - Closed |
| 10 | 630 | 400 | 4 | 1/8" - 1/4" - 3/8" - 1/2" - Closed |
| 20 | 740 | 500 | 4 | 1/8" - 1/4" - 3/8" - 1/2" - Closed |
Bag Models 3D
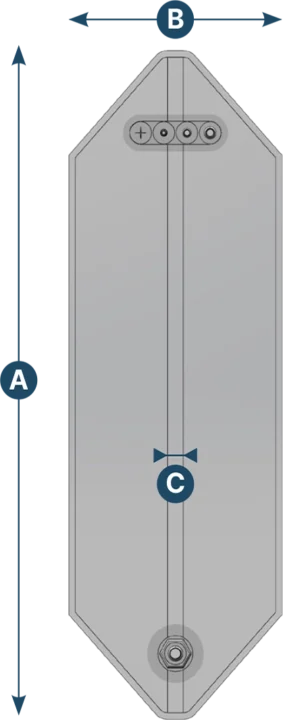
Kolumn 3D bag
| Capacity (L) | A (mm) | B (mm) | C (mm) |
|---|---|---|---|
| 50 | 996 | 315 | 25 |
| 100 | 1101 | 395 | 25 |
| 200 | 1367 | 476 | 25 |
| 300 | 1716 | 515 | 25 |
| 370 | 1698 | 597 | 25 |
| 560 | 2037 | 661 | 25 |
| 750 | 2038 | 757 | 25 |
| 1000 | 2017 | 885 | 25 |
| 1300 | 2212 | 1011 | 25 |
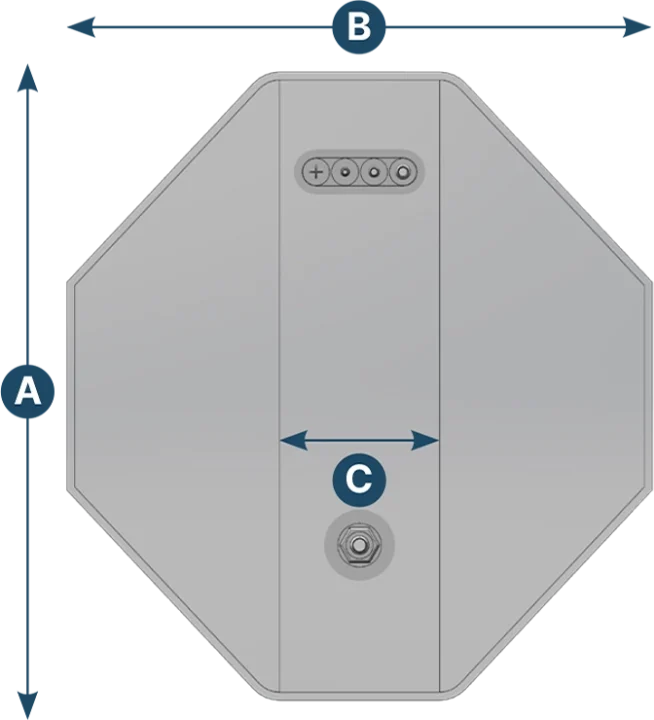
Kontainer 3D bag
| Capacity (L) | A (mm) | B (mm) | C (mm) |
|---|---|---|---|
| 100 | 766 | 715 | 201 |
| 200 | 1009 | 715 | 201 |
| 500 | 1284 | 1105 | 391 |
| 1000 | 1965 | 1102 | 190 |
| 1500 | 2437 | 1090 | 190 |
| 2000 | 2947 | 1090 | 190 |
| 3000 | 3750 | 1102 | 190 |
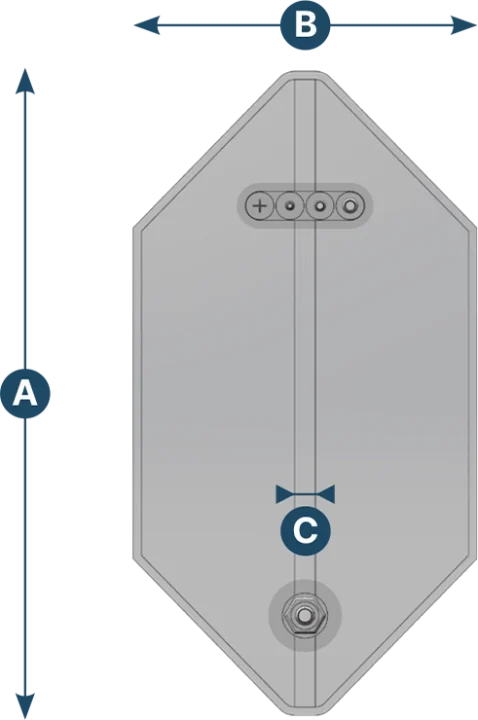
Kube 3D bag
| Capacity (L) | A (mm) | B (mm) | C (mm) |
|---|---|---|---|
| 50 | 765 | 395 | 25 |
| 100 | 927 | 476 | 25 |
| 200 | 1169 | 597 | 25 |
| 400 | 1489 | 757 | 25 |
| 650 | 1750 | 885 | 25 |
| 1000 | 1957 | 991 | 25 |

Get in touch with the team
Our technical department is available to research and study flexible and innovative products in collaboration with the customer.
